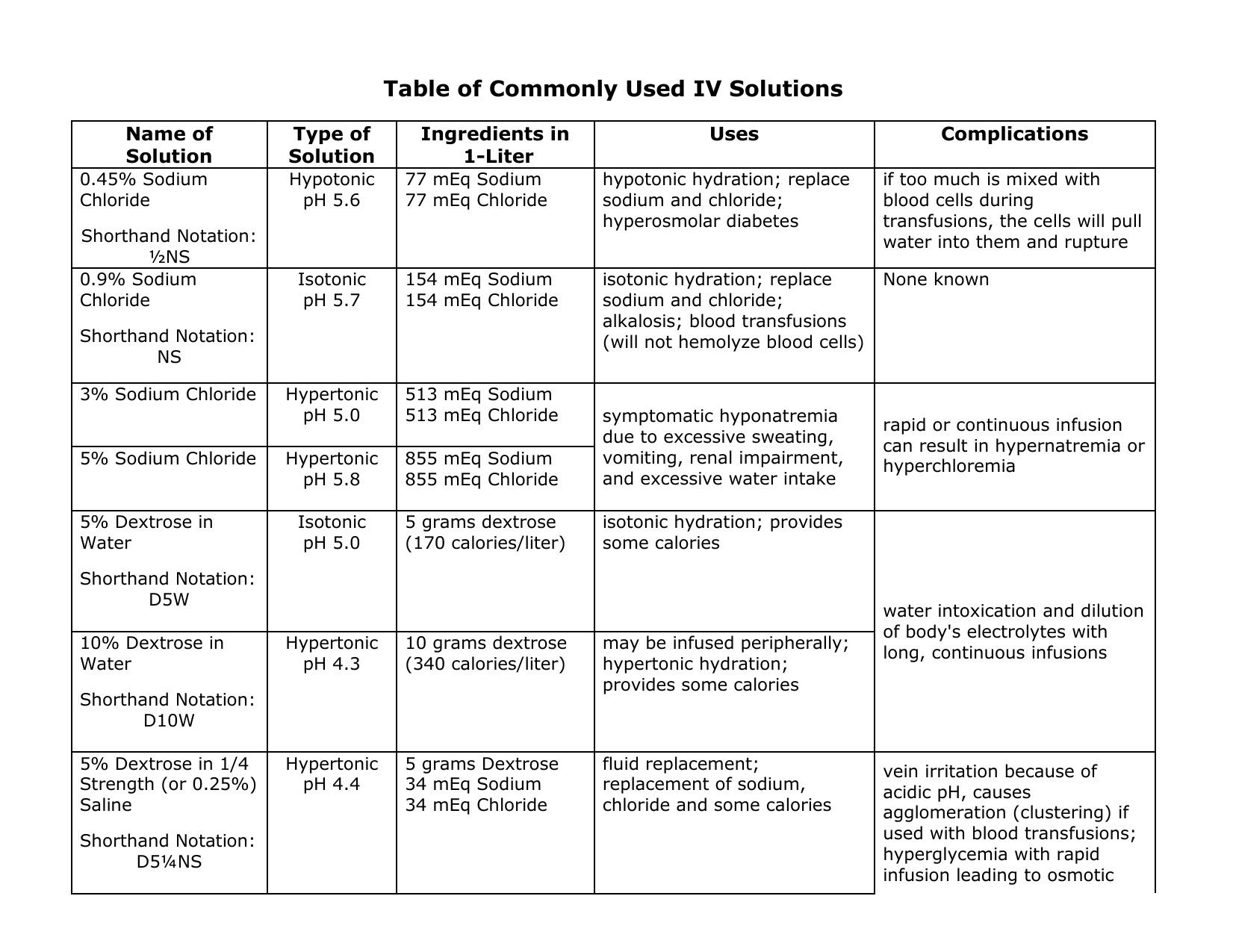
Hypotonic Solutions Iv. Hypotonic is a description of the solute content of one solution in relation to another solution. 5 dextrose in water. Hypotonic iv solution the dextrose solutions come in a variety of concentrations. Iv solutions are considered hypotonic if the total electrolyte content is less than 250 meq l.
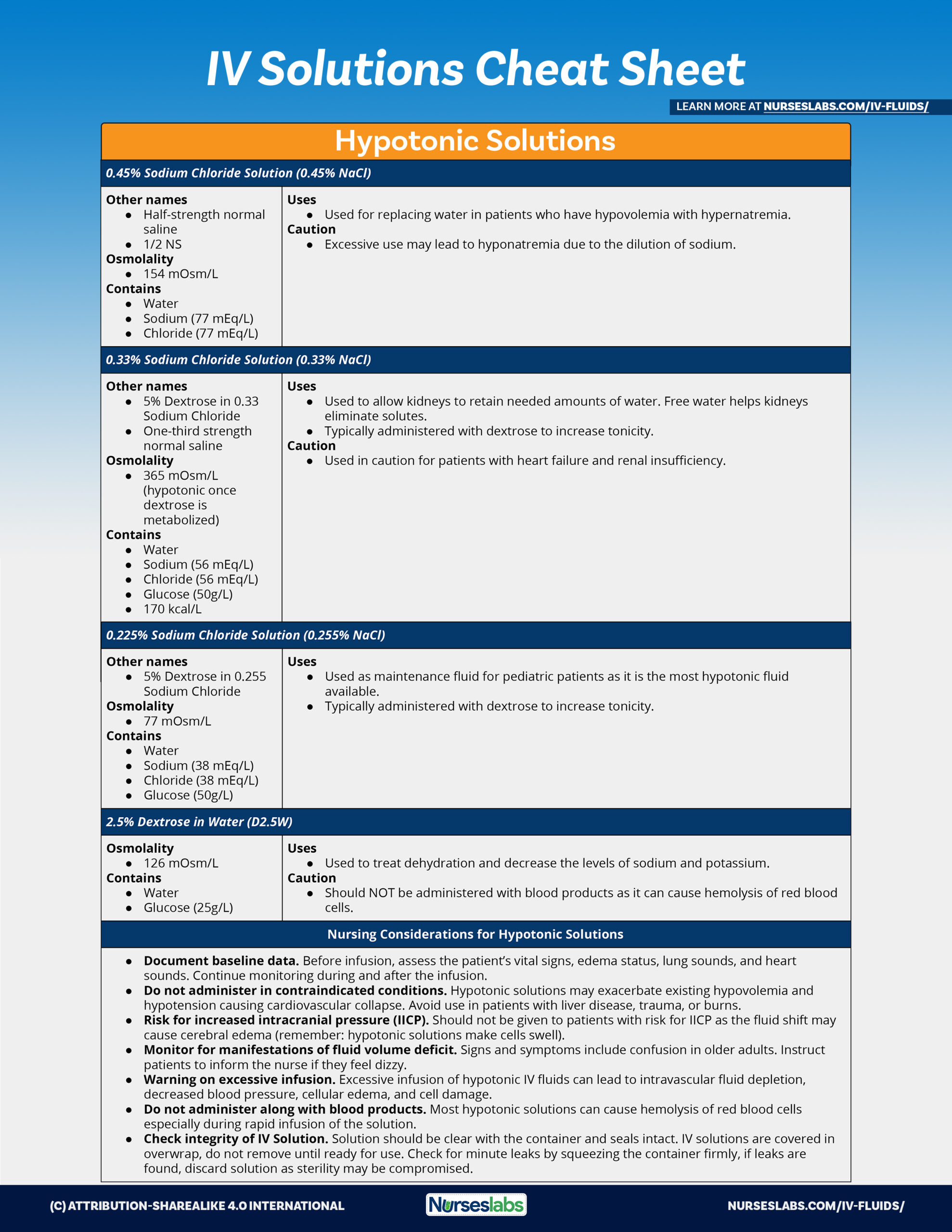
A hypotonic solution is a solution that has a lower solute concentration compared to another solution. Hypotonic solutions are used when the cell is dehydrated and fluids need to be put back intracellularly. Hypotonic is a description of the solute content of one solution in relation to another solution. 45 sodium chloride 0 45 nacl 33 sodium chloride. Some examples are normal saline which has an osmolarity of 154 mosm l but also anything less than that like or normal saline. It is used in biology to help scientist describe cells.
Hypotonic solutions are given for conditions causing intracellular dehydration such as.
Hypotonic solutions are given for conditions causing intracellular dehydration such as. 0 45 sodium chloride 0 45 nacl. The amount of fluid carbohydrate is dependent on the solution. Watch out for depleting the circulatory system of fluid since you are trying to push extracellular fluid into the cell to re hydrate it. Hypotonic is a description of the solute content of one solution in relation to another solution. These solutions provide both fluids and carbohydrates for energy and thus prevent the breakdown of fats and proteins for energy.